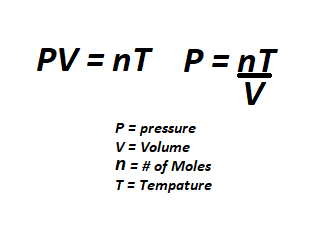Atmospheric Technician: Difference between revisions
No edit summary |
|||
| Line 28: | Line 28: | ||
[[File:Pressure law.png]] | [[File:Pressure law.png]] | ||
All gas can be quantified by its pressure, mole amount, and temperature. These three variables are closely related. | All gas can be quantified by its pressure, mole amount, and temperature. These three variables are closely related. If you have more gas in a given area, the pressure will be larger. The same amount of moles in a smaller area will also mean a higher pressure. If the gas is hot, the pressure will be larger. The opposite is also true, less gas means lower pressure, and colder gas means a lower pressure. Volume also plays a role in pressure. A larger area will require more gas while a smaller area will require less gas to reach the same pressure. Space has a low pressure because the area and temperature are so low. | ||
Moles are a way of measuring how much gas is present in a given area. A higher pressure does not always mean more of a gas in the given area. If you want to physically fit a larger amount of a gas in a specific area, you will need to cool the gas down to lower the temperature, thus lowering the pressure allowing more moles to fit inside the given area. | |||
If you open a canister will 100 moles of air into a large hallway, you will hardly notice a difference in pressure. However, if you open the same tank in a small room the pressure difference will be greater. | |||
The standard livable air requirements are about 20 moles of oxygen and 80 moles of nitrogen at a pressure of 101kpa and tempature of 20° Celsius. If you have less than 20 moles of oxygen present, your character will begin to gasp and take oxygen deprivation damage. If the pressure is any lower or higher than 101kpa, your character will begin to take brute damage in relation to the depressurization or overpressurization levels. If the temperature is much lower or higher than 20°C, you risk your character taking burn damage from the extreme cold or heat. | |||
Generally speaking: | |||
*Hot gas = high pressure. | |||
*Cold gas = low pressure. | |||
*Large hallway = more moles needed to notice pressure change. | |||
*small hallway = less moles needed to notice pressure change. | |||
==Distro== | ==Distro== | ||
Revision as of 21:29, 17 May 2022
WIP
Engineering Department
Atmospheric Technician
- Access: Engineering, Atmospherics, External, Maintenance
- Difficulty: Medium to Hard
- Duties: Manage the station's various gases. Restore breathable atmosphere to depressurized areas. Ensure the station's air remains at livable conditions.
- Supervisors: Chief Engineer, Captain
- Subordinates: None
- Guides: Pipes and vents, Gases, Setting up the mix chamber
Much like your engineering counterpart, the Station Engineer, your job is to fix, maintain, and improve the station's air quality. By working on various pipes, pumps, and vents insure that the hallways remain breathable. Most of the time your job will be to sit back relax in your department until the inevitable hull breach occurs depressurizing the hallways. When this happens don your hardsuit and magboots, turn on your internals, and work with the engineers to patch up the hole. Ensure that the atmosphere in the recently breached area is livable once again with your Gas Analyzer. Once the air quality is back to standards, return to your department to relax or experiment with the pipes until the next inevitable hull breach occurs.
Your supervisor is Chief Engineer.
Atmosia
Atmospherics, or sometimes referred to as Atmosia, is where you will be spending most of your time. In this jungle of pipes and vents is where the magic of atmospherics happens. Make sure to stare at the pipes until they begin to make sense. Observe where the distro and waste loops are and how to keep them separated. Once you feel comfortable with the layout, feel free to tweak the pipes and pump to your liking. Reroute the layout to a more efficient setup or experiment with gases in the mix chamber.
Pressure and moles
All gas can be quantified by its pressure, mole amount, and temperature. These three variables are closely related. If you have more gas in a given area, the pressure will be larger. The same amount of moles in a smaller area will also mean a higher pressure. If the gas is hot, the pressure will be larger. The opposite is also true, less gas means lower pressure, and colder gas means a lower pressure. Volume also plays a role in pressure. A larger area will require more gas while a smaller area will require less gas to reach the same pressure. Space has a low pressure because the area and temperature are so low.
Moles are a way of measuring how much gas is present in a given area. A higher pressure does not always mean more of a gas in the given area. If you want to physically fit a larger amount of a gas in a specific area, you will need to cool the gas down to lower the temperature, thus lowering the pressure allowing more moles to fit inside the given area.
If you open a canister will 100 moles of air into a large hallway, you will hardly notice a difference in pressure. However, if you open the same tank in a small room the pressure difference will be greater.
The standard livable air requirements are about 20 moles of oxygen and 80 moles of nitrogen at a pressure of 101kpa and tempature of 20° Celsius. If you have less than 20 moles of oxygen present, your character will begin to gasp and take oxygen deprivation damage. If the pressure is any lower or higher than 101kpa, your character will begin to take brute damage in relation to the depressurization or overpressurization levels. If the temperature is much lower or higher than 20°C, you risk your character taking burn damage from the extreme cold or heat.
Generally speaking:
- Hot gas = high pressure.
- Cold gas = low pressure.
- Large hallway = more moles needed to notice pressure change.
- small hallway = less moles needed to notice pressure change.
Distro
Distro, or the distribution loop, is usually marked with light blue pipes and is responsible for distributing gas to the station via vents. These pipes start and end in atmospherics and are looped around the entire station. The main purpose of Distro is to ensure that the station maintains a constant pressure of breathable air. The distribution loop works in conjunction with air vents spread across the station to slowly replenish the atmosphere. At round start, Distro will be fully automated and connected to only the nitrogen and oxygen gas chambers.
Waste
Waste, or the waste loop, is usually marked with red pipes and is responsible for removing waste gas around the station via scrubbers. These pipes start and end in atmospherics and are looped around the entire station. The main purpose of the waste loop is the ensure that harmful gases get removed from the station's atmosphere. Scrubbers will remove gas from the hallways and return it to Atmospherics where it will then be separated by gas filters into various holding chambers. Harmful gases will be deposited into their assigned chambers and the breathable air will once again make its way back to Distro and into the station to continue the cycle.
Mix Chamber
The mix chamber is an empty holding area with its own separate loop of pipes and pumps in atmospherics. The mix loop is generally marked with brown pipes and the holding chamber can usually be found close to the external hull, separated from the station by reinforced walls and windows. There will be an emergency button nearby to vent the chamber to space if you need to dump your mix in case an accident arises, or you just wish to reset the chamber for a new mix. The mix chamber is here for you to experiment with different mixes, ratios, temperatures, and pressures while combining gases.
On most stations the Mix chamber will loop around to either the Supermater chamber, to the distro loop, or back to the waste loop. It is generally a good practice to have your mix loop flow back into the waste loop to recycle any unspent gases. You should NEVER have the mix loop flow into distro unless you have a very good reason. Most savvy Atmospherics Technicians physically disconnect the mix loop from distro at round start to prevent an easy sabotage target or accident from occurring later in the round.
See the guide to Gases if you are interested to learn how different gases interact with each other at different temperatures and pressures.
Gas Miner
Gas miners create new gas from nothing and are solely used to make sure the station has an infinite amount of a specific gas. Gas Miners come in different types and create different types of gas. A oxygen miner will create oxygen gas while a plasma miner will create plasma gas. Gas Miners can be found in Atmospherics inside each gas holding chamber.
Gas holding chambers

Located in atmospherics is a series of separated gas holding chambers. These chambers will generally contain a specific gas and its respective gas miner. Depending on the station layout, the holding chambers may be separated from the hull by reinforced walls and windows. If a holding chamber is breached all the gas held inside will be vented out to space. Patch up the breach to allow the miner to replenish the storage of gas.
Each chamber will be connected to the waste and mix loops. The nitrogen and oxygen chambers will also be connected to the distro loop. The gas can be extracted using pumps and mixers and sent off to various locations around atmospherics. Unused gas in the system will be recycled as it flows back along the waste loop. filters will separate each gas and pump it into its assigned chamber. These chambers can generally be considered an infinite source of gas.
Each chamber will have a sign on the wall displaying information on what gas is in its own specific chamber. Each chamber should contain pure gas of its element and can safely be treated as such.
Useful trivia and tricks
- always be prepared to fix a breach. Carry your Atmospherics hardsuit or firesuit with you so you can survive long enough to fix any breach at a moments notice.
- the Atmospherics firesuit is space proof and functions like a hardsuit. Just make sure you wear the helmet with it or else it wont give you any protection.
- The standard air mix is about 20% oxygen and 80% nitrogen @ 101.7 kPa at 293.2K (20°C).
- Overpressurization can be just as harmful as depressurization!
- A pressurized pipe will violently decompress if unwrenched!
- If you want to check the pressure and temperature of a pipe, hold your gas analyzer in hand, shift left-click on the pipe, and click on the magnifying glass in pop-up window.
- Make sure that the distro loop never connects to anything else like the mix or waste loop. unless you want to flood the station with superheated plasma and risk getting an angry admin message.